

For pain and fever in cattle, relief is in the palm of your hand
FDA-approved for control of fever due to mastitis.
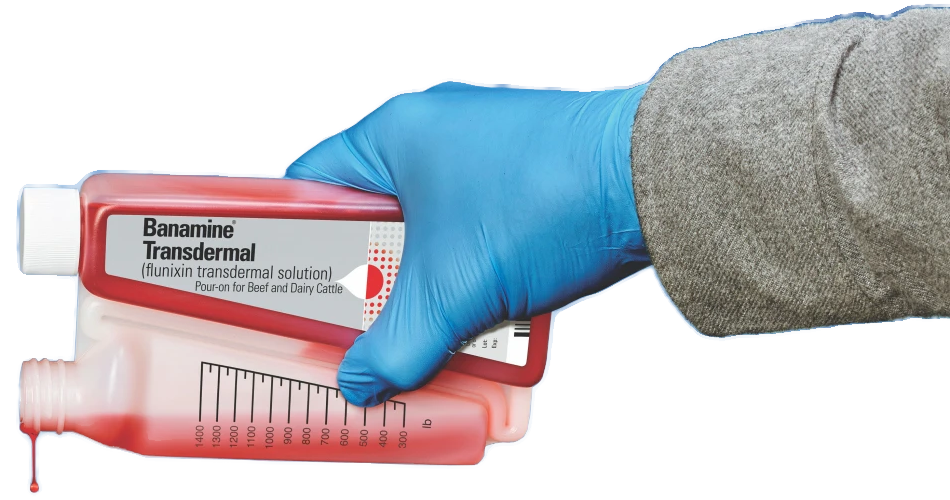
The first FDA-approved pour-on for pain
and fever control in cattle
Using pour-on application to reduce fever in cattle caused by BRD or acute mastitis and relieve pain due to foot rot aligns with industry efforts to continuously improve animal care and mitigate pain.

GOES TO WORK
IN A HURRY
BANAMINE TRANSDERMAL controls pain and fever quickly
EASY
TO APPLY
You can make quick work of treatment with this simple pour-on.
EASY
ON CATTLE
Application requires less handling, resulting in less stress on the animals.
BQA
FRIENDLY
Pour-on application eliminates injection-site lesions.
Control pain and fever in cattle.
Pain and fever can cause cattle to go off feed. But now, easy-to-use BANAMINE® TRANSDERMAL (flunixin transdermal solution) helps get ‘em back where they belong.
FDA-approved for control of fever associated with bovine respiratory disease and acute bovine mastitis, and the control of pain associated with foot rot, BANAMINE TRANSDERMAL is the first and only non-steroidal anti-inflammatory (NSAID) cattle product available with a convenient pour-on route of administration.
Overview of BANAMINE TRANSDERMAL
IMPORTANT SAFETY INFORMATION:
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. Milk that has been taken during treatment and for 48 hours after treatment must not be used for human consumption. Cattle must not be slaughtered for human consumption within 8 days of the last treatment. Not for use in replacement dairy heifers 20 months of age or older or dry dairy cows; use in these cattle may cause drug residues in milk and/or calves born to these cows or heifers. Not for use in beef and dairy bulls intended for breeding over 1 year of age, beef calves less than 2 months of age, dairy calves, and veal calves. Do not use within 48 hours of expected parturition. Approved only as a single topical dose in cattle. For complete information on BANAMINE® TRANSDERMAL, see accompanying product package insert.
